What Type Of Rock Forms When Heat And Pressure Change The Chemical Makeup Of A Rock
| Sedimentary stone | |
 Limestone outcrop in the Torcal de Antequera nature reserve of Málaga, Spain | |
| Composition | |
|---|---|
| Calcium carbonate: inorganic crystalline calcite or organic calcareous fabric |
Limestone is a mutual type of carbonate sedimentary stone which is the main source of the textile lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate (CaCO3 ). Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can have place through both biological and nonbiological processes, though biological processes, such equally the accumulation of corals and shells in the sea, have likely been more important for the terminal 540 million years.[1] [2] Limestone oft contains fossils, and these provide scientists with information on ancient environments and on the evolution of life.[three]
About xx% to 25% of sedimentary rock is carbonate rock, and near of this is limestone.[4] [three] The remaining carbonate rock is more often than not dolomite, a closely related stone, which contains a high percentage of the mineral dolomite, CaMg(COiii)two . Magnesian limestone is an obsolete and poorly-defined term used variously for dolomite, for limestone containing significant dolomite (dolomitic limestone), or for any other limestone containing a significant percentage of magnesium.[5] Well-nigh limestone was formed in shallow marine environments, such as continental shelves or platforms, though smaller amounts were formed in many other environments. Much dolomite is secondary dolomite, formed by chemic alteration of limestone.[6] [7] Limestone is exposed over large regions of the Earth's surface, and because limestone is slightly soluble in rainwater, these exposures oft are eroded to become karst landscapes. Most cavern systems are found in limestone bedrock.
Limestone has numerous uses: as a building cloth, an essential component of physical (Portland cement), as aggregate for the base of roads, as white pigment or filler in products such as toothpaste or paints, as a chemic feedstock for the production of lime, equally a soil conditioner, and as a popular decorative addition to stone gardens. Limestone formations contain about 30% of the world'due south petroleum reservoirs.[3]
Description [edit]

Limestone is composed mostly of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate (CaCO3 ). Dolomite, CaMg(CO3)2 , is an uncommon mineral in limestone, and siderite or other carbonate minerals are rare. Withal, the calcite in limestone often contains a few percent of magnesium. Calcite in limestone is divided into depression-magnesium and high-magnesium calcite, with the dividing line placed at a limerick of four% magnesium. Loftier-magnesium calcite retains the calcite mineral structure, which is singled-out from dolomite. Aragonite does non commonly comprise pregnant magnesium.[8] Most limestone is otherwise chemically adequately pure, with clastic sediments (mainly fine-grained quartz and dirt minerals) making upwards less than 5%[9] to x%[10] of the limerick. Organic affair typically makes up around 0.2% of a limestone and rarely exceeds 1%.[11]
Limestone often contains variable amounts of silica in the form of chert or siliceous skeletal fragments (such as sponge spicules, diatoms, or radiolarians).[12] Fossils are too mutual in limestone.[3]
Limestone is commonly white to greyness in color. Limestone that is unusually rich in organic matter can be almost black in color, while traces of fe or manganese can requite limestone an off-white to yellow to cerise color. The density of limestone depends on its porosity, which varies from 0.1% for the densest limestone to 40% for chalk. The density correspondingly ranges from 1.v to ii.7 m/cm3. Although relatively soft, with a Mohs hardness of 2 to 4, dumbo limestone can have a burdensome forcefulness of upwards to 180 MPa.[thirteen] For comparison, concrete typically has a crushing strength of virtually 40 MPa.[14]
Although limestones prove little variability in mineral composition, they prove keen variety in texture.[15] Even so, almost limestone consists of sand-sized grains in a carbonate mud matrix. Because limestones are often of biological origin and are normally composed of sediment that is deposited close to where it formed, nomenclature of limestone is usually based on its grain type and mud content.[9]
Grains [edit]


Ooids in limestone of the Carmel Formation (Middle Jurassic) of southwestern Utah.

Thin-section view of a Middle Jurassic limestone in southern Utah, U.S. The circular grains are ooids; the largest is 1.2 mm (0.05 in) in diameter. This limestone is an oosparite.
Most grains in limestone are skeletal fragments of marine organisms such equally coral or foraminifera.[16] These organisms secrete structures made of aragonite or calcite, and exit these structures behind when they die. Other carbonate grains composing limestones are ooids, peloids, and limeclasts (intraclasts and extraclasts).[17]
Skeletal grains have a composition reflecting the organisms that produced them and the environment in which they were produced.[18] Low-magnesium calcite skeletal grains are typical of articulate brachiopods, planktonic (free-floating) foraminifera, and coccoliths. High-magnesium calcite skeletal grains are typical of benthic (bottom-habitation) foraminifera, echinoderms, and coralline algae. Aragonite skeletal grains are typical of molluscs, calcareous green algae, stromatoporoids, corals, and tube worms. The skeletal grains also reflect specific geological periods and environments. For example, coral grains are more common in high-energy environments (characterized by strong currents and turbulence) while bryozoan grains are more mutual in depression-energy environments (characterized by tranquility h2o).[19]
Ooids (sometimes called ooliths) are sand-sized grains (less than 2mm in diameter) consisting of i or more layers of calcite or aragonite around a central quartz grain or carbonate mineral fragment. These probable form past directly precipitation of calcium carbonate onto the ooid. Pisoliths are similar to ooids, but they are larger than 2mm in diameter and tend to be more irregular in shape. Limestone composed mostly of ooids is called an oolite or sometimes an oolitic limestone. Ooids form in high-energy environments, such equally the Bahama platform, and oolites typically testify crossbedding and other features associated with degradation in strong currents.[xx] [21]
Oncoliths resemble ooids but show a radial rather than layered internal construction, indicating that they were formed by algae in a normal marine environment.[twenty]
Peloids are structureless grains of microcrystalline carbonate probable produced past a diverseness of processes.[22] Many are idea to exist fecal pellets produced by marine organisms. Others may be produced by endolithic (wearisome) algae[23] or other microorganisms[24] or through breakdown of mollusc shells.[25] They are hard to encounter in a limestone sample except in thin section and are less common in ancient limestones, possibly because compaction of carbonate sediments disrupts them.[23]
Limeclasts are fragments of existing limestone or partially lithified carbonate sediments. Intraclasts are limeclasts that originate close to where they are deposited in limestone, while extraclasts come from outside the depositional area. Intraclasts include grapestone, which is clusters of peloids cemented together by organic material or mineral cement. Extraclasts are uncommon, are usually accompanied by other clastic sediments, and indicate degradation in a tectonically active area or as role of a turbidity current.[26]
Mud [edit]
The grains of most limestones are embedded in a matrix of carbonate mud. This is typically the largest fraction of an ancient carbonate rock.[23] Mud consisting of individual crystals less than 5 microns in length is described as micrite.[27] In fresh carbonate mud, micrite is more often than not pocket-sized aragonite needles, which may precipitate directly from seawater,[28] be secreted by algae,[29] or be produced by abrasion of carbonate grains in a loftier-free energy environment.[30] This is converted to calcite within a few million years of degradation. Further recrystallization of micrite produces microspar, with grains from v to 15 microns in bore.[28]
Limestone often contains larger crystals of calcite, ranging in size from 0.02 to 0.i mm, that are described as sparry calcite or sparite. Sparite is distinguished from micrite past a grain size of over 20 microns and because sparite stands out nether a hand lens or in thin section every bit white or transparent crystals. Sparite is distinguished from carbonate grains past its lack of internal structure and its feature crystal shapes. [31]
Geologists are careful to distinguish between sparite deposited equally cement and sparite formed past recrystallization of micrite or carbonate grains. Sparite cement was likely deposited in pore infinite between grains, suggesting a high-energy depositional environment that removed carbonate mud. Recrystallized sparite is not diagnostic of depositional environment.[31]
Other characteristics [edit]

Limestone outcrops are recognized in the field by their softness (calcite and aragonite both have a Mohs hardness of less than four, well below mutual silicate minerals) and considering limestone bubbles vigorously when a drop of dilute hydrochloric acid is dropped on information technology. Dolomite is besides soft but reacts only feebly with dilute hydrochloric acid, and information technology usually weathers to a characteristic dull yellowish-brown colour due to the presence of ferrous iron. This is released and oxidized equally the dolomite weathers.[9] Impurities (such every bit clay, sand, organic remains, fe oxide, and other materials) will crusade limestones to showroom different colors, especially with weathered surfaces.
The makeup of a carbonate stone outcrop can be estimated in the field by etching the surface with dilute hydrochloric acid. This etches away the calcite and aragonite, leaving behind any silica or dolomite grains. The latter can exist identified by their rhombohedral shape.[nine]
Crystals of calcite, quartz, dolomite or barite may line small cavities (vugs) in the rock. Vugs are a form of secondary porosity, formed in existing limestone by a alter in environment that increases the solubility of calcite.[32]
Dense, massive limestone is sometimes described as "marble". For example, the famous Portoro "marble" of Italy is really a dense black limestone.[33] True marble is produced by recrystallization of limestone during regional metamorphism that accompanies the mountain edifice process (orogeny). It is distinguished from dense limestone by its coarse crystalline texture and the formation of distinctive minerals from the silica and dirt present in the original limestone.[34]
Classification [edit]


2 major classification schemes, the Folk and Dunham, are used for identifying the types of carbonate rocks collectively known as limestone.
Folk classification [edit]
Robert Fifty. Folk adult a nomenclature system that places primary accent on the detailed composition of grains and interstitial material in carbonate rocks.[35] Based on limerick, there are three master components: allochems (grains), matrix (by and large micrite), and cement (sparite). The Folk system uses two-function names; the first refers to the grains and the second to the cement. For instance, a limestone consisting mainly of ooids, with a crystalline matrix, would be termed an oosparite. Information technology is helpful to have a petrographic microscope when using the Folk scheme, because it is easier to determine the components present in each sample.[36]
Dunham nomenclature [edit]
Robert J. Dunham published his organization for limestone in 1962. It focuses on the depositional fabric of carbonate rocks. Dunham divides the rocks into iv main groups based on relative proportions of coarser clastic particles, based on criteria such as whether the grains were originally in mutual contact, and therefore self-supporting, or whether the rock is characterized by the presence of frame builders and algal mats. Unlike the Folk scheme, Dunham deals with the original porosity of the rock. The Dunham scheme is more useful for hand samples considering it is based on texture, non the grains in the sample.[37]
A revised nomenclature was proposed by Wright (1992). Information technology adds some diagenetic patterns to the classification scheme.[38]
Other descriptive terms [edit]
Travertine is a term applied to calcium carbonate deposits formed in freshwater environments, particularly hot springs. Such deposits are typically massive, dumbo, and banded. When the deposits are highly porous, and then that they accept a spongelike texture, they are typically described as tufa. Secondary calcite deposited past supersaturated meteoric waters (groundwater) in caves is also sometimes described every bit travertine. This produces speleothems, such as stalagmites and stalactites.[39]
Coquina is a poorly consolidated limestone composed of abraded pieces of coral, shells, or other fossil droppings. When better consolidated, information technology is described equally coquinite.[40]
Chalk is a soft, earthy, fine-textured limestone equanimous of the tests of planktonic microorganisms such as foraminifera, while marl is an bawdy mixture of carbonates and silicate sediments.[xl]
Formation [edit]
Limestone forms when calcite or aragonite precipitate out of h2o containing dissolved calcium, which can take place through both biological and nonbiological processes.[41] The solubility of calcium carbonate (CaCO3 ) is controlled largely by the amount of dissolved carbon dioxide (COtwo ) in the water. This is summarized in the reaction:
-
- CaCO3 + HiiO + CO2 → Ca2+ + 2HCO − 3
Increases in temperature or decreases in pressure level tend to reduce the corporeality of dissolved COii and precipitate CaCO3 . Reduction in salinity also reduces the solubility of CaCO3 , past several orders of magnitude for fresh h2o versus seawater. [42]
Virtually-surface water of the earth'south oceans are oversaturated with CaCOthree by a factor of more than vi.[43] The failure of CaCO3 to apace precipitate out of these waters is likely due to interference by dissolved magnesium ions with nucleation of calcite crystals, the necessary offset step in atmospheric precipitation. Precipitation of aragonite may be suppressed by the presence of naturally occurring organic phosphates in the h2o. Although ooids likely class through purely inorganic processes, the bulk of CaCO3 precipitation in the oceans is the upshot of biological activity.[44] Much of this takes place on carbonate platforms.
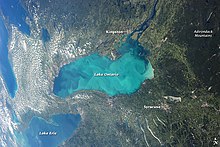
An aerial view of a whiting event precipitation cloud in Lake Ontario.
The origin of carbonate mud,[xxx] and the processes past which it is converted to micrite,[45] continue to be a subject of inquiry. Modern carbonate mud is composed mostly of aragonite needles effectually five microns in length. Needles of this shape and composition are produced by calcareous algae such as Penicillus, making this a plausible source of mud.[46] Some other possibility is directly precipitation from the h2o. A phenomenon known as whitings occurs in shallow waters, in which white streaks containing dispersed micrite appear on the surface of the water. It is uncertain whether this is freshly precipitated aragonite or only material stirred up from the bottom, only there is some evidence that whitings are caused past biological precipitation of aragonite every bit part of a bloom of cyanobacteria or microalgae.[47] However, stable isotope ratios in modern carbonate mud appear to be inconsistent with either of these mechanisms, and abrasion of carbonate grains in high-energy environments has been put forward as a third possibility.[thirty]
Formation of limestone has likely been dominated by biological processes throughout the Phanerozoic, the final 540 million years of the World's history. Limestone may accept been deposited by microorganisms in the Precambrian, prior to 540 million years ago, only inorganic processes were probably more important and likely took place in an ocean more highly oversaturated in calcium carbonate than the modernistic ocean.[48]
Diagenesis [edit]
Diagenesis is the process in which sediments are compacted and turned into solid rock. During diagenesis of carbonate sediments, significant chemical and textural changes accept place. For example, aragonite is converted to depression-magnesium calcite. Diagenesis is the probable origin of pisoliths, concentrically layered particles ranging from one to ten millimeters (0.039 to 0.394 in) in bore constitute in some limestones. Pisoliths superficially resemble ooids but take no nucleus of foreign matter, fit together tightly, and bear witness other signs that they formed afterward the original deposition of the sediments.[49]

Akcakoca chert nodules within soft limestone

Silicification occurs early in diagenesis, at low pH and temperature, and contributes to fossil preservation. Silicification takes place through the reaction:
-
- CaCOthree + H2O + COii + H4SiO4 → SiO2 + Ca2+ + 2HCO − 3 + 2 H2O
Fossils are often preserved in exquisite detail every bit chert.[fifty]
Cementing takes identify apace in carbonate sediments, typically inside less than a meg years of deposition. Some cementing occurs while the sediments are nonetheless under water, forming hardgrounds. Cementing accelerates afterwards the retreat of the body of water from the depositional environment, as rainwater infiltrates the sediment beds, often within just a few grand years. As rainwater mixes with groundwater, aragonite and loftier-magnesium calcite are converted to low-calcium calcite. Cementing of thick carbonate deposits past rainwater may commence fifty-fifty before the retreat of the sea, equally rainwater can infiltrate over 100 kilometers (60 mi) into sediments beneath the continental shelf.[51]
As carbonate sediments are increasingly deeply buried under younger sediments, chemical and mechanical compaction of the sediments increases. Chemic compaction takes place by pressure solution of the sediments. This process dissolves minerals from points of contact betwixt grains and redeposits information technology in pore infinite, reducing the porosity of the limestone from an initial high value of 40% to fourscore% to less than ten%.[52] Pressure level solution produces distinctive styolites, irregular surfaces within the limestone at which silica-rich sediments accumulate. These may reverberate dissolution and loss of a considerable fraction of the limestone bed. At depths greater than i kilometer (0.62 mi), burial cementation completes the lithification process. Burial cementation does non produce styolites.[53]
When overlying beds are eroded, bringing limestone closer to the surface, the final stage of diagenesis takes place. This produces secondary porosity as some of the cement is dissolved past rainwater infiltrating the beds. This may include the formation of vugs, which are crystal-lined cavities within the limestone.[53]
Diagenesis may include conversion of limestone to dolomite by magnesium-rich fluids. There is considerable testify of replacement of limestone past dolomite, including sharp replacement boundaries that cutting across bedding.[54] The procedure of dolomitization remains an area of agile research,[55] merely possible mechanisms include exposure to concentrated brines in hot environments (evaporative reflux) or exposure to diluted seawater in delta or estuary environments (Dorag dolomitization).[56] However, Dorag dolomitization has fallen into disfavor equally a mechanism for dolomitization,[57] with one 2004 review paper describing it bluntly as "a myth".[55] Ordinary seawater is capable of converting calcite to dolomite, if the seawater is regularly flushed through the rock, every bit by the ebb and flow of tides (tidal pumping).[54] Once dolomitization begins, information technology proceeds apace, and then that at that place is very footling carbonate rock containing mixed calcite and dolomite. Carbonate rock tends to be either almost all calcite/aragonite or almost all dolomite.[56]
Occurrence [edit]
About 20% to 25% of sedimentary rock is carbonate stone,[3] and most of this is limestone.[17] [3] Limestone is plant in sedimentary sequences as quondam as 2.7 billion years.[58] However, the compositions of carbonate rocks testify an uneven distribution in fourth dimension in the geologic record. About 95% of modernistic carbonates are composed of loftier-magnesium calcite and aragonite.[59] The aragonite needles in carbonate mud are converted to depression-magnesium calcite within a few million years, as this is the well-nigh stable form of calcium carbonate.[28] Ancient carbonate formations of the Precambrian and Paleozoic contain abundant dolomite, but limestone dominates the carbonate beds of the Mesozoic and Cenozoic. Modern dolomite is quite rare. In that location is evidence that, while the modern body of water favors precipitation of aragonite, the oceans of the Paleozoic and heart to late Cenozoic favored precipitation of calcite. This may indicate a lower Mg/Ca ratio in the sea water of those times.[60] This magnesium depletion may be a consequence of more than rapid body of water floor spreading, which removes magnesium from ocean water. The modern sea and the ocean of the Mesozoic accept been described as "aragonite seas".[61]
Most limestone was formed in shallow marine environments, such as continental shelves or platforms. Such environments form but about v% of the sea basins, just limestone is rarely preserved in continental gradient and deep bounding main environments. The all-time environments for degradation are warm waters, which have both a high organic productivity and increased saturation of calcium carbonate due to lower concentrations of dissolved carbon dioxide. Modern limestone deposits are virtually always in areas with very little silica-rich sedimentation, reflected in the relative purity of virtually limestones. Reef organisms are destroyed by muddy, stagnant river water, and carbonate grains are ground down past much harder silicate grains.[62] Unlike clastic sedimentary rock, limestone is produced almost entirely from sediments originating at or near the place of deposition.[63]

Limestone formations tend to evidence precipitous changes in thickness. Large moundlike features in a limestone formation are interpreted as ancient reefs, which when they appear in the geologic record are called bioherms. Many are rich in fossils, merely most lack whatever connected organic framework like that seen in modern reefs. The fossil remains are present as split fragments embedded in ample mud matrix. Much of the sedimentation shows indications of occurring in the intertidal or supratidal zones, suggesting sediments rapidly make full available adaptation space in the shelf or platform.[64] Deposition is too favored on the seaward margin of shelves and platforms, where there is upwelling deep ocean water rich in nutrients that increase organic productivity. Reefs are common here, but when lacking, ooid shoals are establish instead. Finer sediments are deposited close to shore.[65]
The lack of deep sea limestones is due in office to rapid subduction of oceanic crust, but is more a consequence of dissolution of calcium carbonate at depth. The solubility of calcium carbonate increases with pressure level and fifty-fifty more than with higher concentrations of carbon dioxide, which is produced by decomposable organic affair settling into the deep ocean that is not removed by photosynthesis in the dark depths. As a effect, there is a fairly sharp transition from water saturated with calcium carbonate to water unsaturated with calcium carbonate, the lysocline, which occurs at the calcite compensation depth of 4,000 to 7,000 meters (13,000 to 23,000 ft). Below this depth, foraminifera tests and other skeletal particles apace dissolve, and the sediments of the body of water floor abruptly transition from carbonate ooze rich in foraminifera and coccolith remains (Globigerina ooze) to silicic mud defective carbonates.[66]

In rare cases, turbidites or other silica-rich sediments coffin and preserve benthic (deep ocean) carbonate deposits. Ancient benthic limestones are microcrystalline and are identified past their tectonic setting. Fossils typically are foraminifera and coccoliths. No pre-Jurassic benthic limestones are known, probably because carbonate-shelled plankton had non yet evolved.[67]
Limestones also class in freshwater environments.[68] These limestones are not unlike marine limestone, merely accept a lower multifariousness of organisms and a greater fraction of silica and clay minerals characteristic of marls. The Green River Germination is an case of a prominent freshwater sedimentary germination containing numerous limestone beds.[69] Freshwater limestone is typically micritic. Fossils of charophyte (stonewort), a class of freshwater light-green algae, are characteristic of these environments, where the charophytes produce and trap carbonates.[seventy]
Limestones may also form in evaporite depositional environments.[71] [72] Calcite is one of the first minerals to precipitate in marine evaporites.[73]
Limestone and living organisms [edit]

Nigh limestone is formed by the activities of living organisms near reefs, merely the organisms responsible for reef germination have inverse over geologic time. For case, stromatolites are mound-shaped structures in ancient limestones, interpreted as colonies of cyanobacteria that accumulated carbonate sediments, but stromatolites are rare in younger limestones.[74] Organisms precipitate limestone both directly as part of their skeletons, and indirectly by removing carbon dioxide from the water by photosynthesis and thereby decreasing the solubility of calcium carbonate.[seventy]
Limestone shows the same range of sedimentary structures establish in other sedimentary rocks. However, effectively structures, such as lamination, are often destroyed past the burrowing activities of organisms (bioturbation). Fine lamination is characteristic of limestone formed in playa lakes, which lack the burrowing organisms.[75] Limestones also show distinctive features such as geopetal structures, which form when curved shells settle to the lesser with the concave face downwardly. This traps a void space that can afterward be filled by sparite. Geologists employ geopetal structures to determine which direction was upward at the time of degradation, which is non always obvious with highly plain-featured limestone formations.[76]
The cyanobacterium Hyella balani tin can bore through limestone; as can the greenish alga Eugamantia sacculata and the fungus Ostracolaba implexa.[77]
Micritic mud mounds [edit]
Micricitic mud mounds are subcircular domes of micritic calcite that lacks internal structure. Mod examples are upwards to several hundred meters thick and a kilometer across, and have steep slopes (with slope angles of around l degrees). They may be composed of peloids swept together by currents and stabilized by Thallasia grass or mangroves. Bryozoa may also contribute to mound germination past helping to trap sediments.[78]
Mud mounds are plant throughout the geologic record, and prior to the early Ordovician, they were the dominant reef type in both deep and shallow water. These mud mounds probable are microbial in origin. Following the appearance of frame-edifice reef organisms, mud mounds were restricted mainly to deeper water.[79]
Organic reefs [edit]
Organic reefs course at depression latitudes in shallow water, not more than a few meters deep. They are complex, diverse structures found throughout the fossil tape. The frame-building organisms responsible for organic reef formation are characteristic of different geologic fourth dimension periods: Archaeocyathids appeared in the early Cambrian; these gave fashion to sponges by the late Cambrian; subsequently successions included stromatoporoids, corals, algae, bryozoa, and rudists (a grade of bivalve mollusc).[80] [81] [82] The extent of organic reefs has varied over geologic time, and they were likely most extensive in the middle Devonian, when they covered an area estimated at 5,000,000 square kilometers (1,900,000 sq mi). This is roughly x times the extent of modern reefs. The Devonian reefs were constructed largely past stromatoporoids and tabulate corals, which were devastated by the late Devonian extinction.[83]
Organic reefs typically have a complex internal structure. Whole body fossils are usually arable, but ooids and interclasts are rare inside the reef. The cadre of a reef is typically massive and unbedded, and is surrounded by a talus that is greater in book than the core. The talus contains abundant intraclasts and is normally either floatstone, with x% or more of grains over 2mm in size embedded in abundant matrix, or rudstone, which is mostly large grains with sparse matrix. The talus grades to planktonic fine-grained carbonate mud, and so noncarbonate mud away from the reef.[80]
Limestone mural [edit]



Limestone is partially soluble, particularly in acrid, and therefore forms many erosional landforms. These include limestone pavements, pot holes, cenotes, caves and gorges. Such erosion landscapes are known equally karsts. Limestone is less resistant to erosion than nearly igneous rocks, simply more resistant than most other sedimentary rocks. It is therefore usually associated with hills and downland, and occurs in regions with other sedimentary rocks, typically clays.[84] [85]
Karst regions overlying limestone bedrock tend to have fewer visible to a higher place-ground sources (ponds and streams), as surface water easily drains downwards through joints in the limestone. While draining, h2o and organic acid from the soil slowly (over thousands or millions of years) enlarges these cracks, dissolving the calcium carbonate and conveying it away in solution. Virtually cave systems are through limestone bedrock. Cooling groundwater or mixing of different groundwaters will also create conditions suitable for cave germination.[84]
Coastal limestones are often eroded by organisms which bore into the rock by various means. This procedure is known as bioerosion. Information technology is most mutual in the tropics, and information technology is known throughout the fossil record.[86]
Bands of limestone emerge from the Globe'southward surface in often spectacular rocky outcrops and islands. Examples include the Stone of Gibraltar,[87] the Burren in County Clare, Ireland;[88] Malham Cove in North Yorkshire and the Isle of Wight,[89] England; the Great Orme in Wales;[90] on Fårö near the Swedish island of Gotland,[91] the Niagara Escarpment in Canada/United states of america;[92] Notch Superlative in Utah;[93] the Ha Long Bay National Park in Vietnam;[94] and the hills around the Lijiang River and Guilin city in China.[95]
The Florida Keys, islands off the south coast of Florida, are composed mainly of oolitic limestone (the Lower Keys) and the carbonate skeletons of coral reefs (the Upper Keys), which thrived in the expanse during interglacial periods when sea level was higher than at present.[96]
Unique habitats are found on alvars, extremely level expanses of limestone with thin soil mantles. The largest such area in Europe is the Stora Alvaret on the island of Öland, Sweden.[97] Another area with big quantities of limestone is the island of Gotland, Sweden.[98] Huge quarries in northwestern Europe, such equally those of Mount Saint Peter (Belgium/Netherlands), extend for more than than a hundred kilometers.[99]
Uses [edit]


Limestone is a raw material that is used globally in a variety of different ways including construction, agronomics and equally industrial materials.[101] Limestone is very common in compages, especially in Europe and North America. Many landmarks across the world, including the Dandy Pyramid and its associated complex in Giza, Egypt, were made of limestone. So many buildings in Kingston, Ontario, Canada were, and go along to be, synthetic from information technology that it is nicknamed the 'Limestone Metropolis'.[102] Limestone, metamorphosed by oestrus and pressure produces marble, which has been used for many statues, buildings and stone tabletops.[103] On the island of Malta, a variety of limestone called Globigerina limestone was, for a long fourth dimension, the simply building textile available, and is still very often used on all types of buildings and sculptures.[104]
Limestone can exist processed into many various forms such as brick, cement, powdered/crushed, or as a filler.[105] Limestone is readily bachelor and relatively easy to cut into blocks or more elaborate etching.[100] Ancient American sculptors valued limestone because it was easy to piece of work and good for fine detail. Going back to the Late Preclassic period (by 200–100 BCE), the Maya culture (Aboriginal United mexican states) created refined sculpture using limestone considering of these excellent carving properties. The Maya would decorate the ceilings of their sacred buildings (known as lintels) and embrace the walls with carved limestone panels. Carved on these sculptures were political and social stories, and this helped communicate messages of the king to his people.[106] Limestone is long-lasting and stands up well to exposure, which explains why many limestone ruins survive. However, information technology is very heavy (density 2.vi[107]), making it impractical for alpine buildings, and relatively expensive as a building cloth.
Limestone was most popular in the late 19th and early on 20th centuries. Railroad train stations, banks and other structures from that era were normally made of limestone. It is used as a facade on some skyscrapers, but only in thin plates for covering, rather than solid blocks. In the United States, Indiana, most notably the Bloomington expanse, has long been a source of high-quality quarried limestone, called Indiana limestone. Many famous buildings in London are congenital from Portland limestone. Houses congenital in Odessa in Ukraine in the 19th century were mostly constructed from limestone and the extensive remains of the mines now form the Odessa Catacombs.[108]
Limestone was also a very popular building block in the Centre Ages in the areas where information technology occurred, since it is hard, durable, and commonly occurs in easily attainable surface exposures. Many medieval churches and castles in Europe are made of limestone. Beer stone was a popular kind of limestone for medieval buildings in southern England.[109]
-
Cutting limestone blocks at a quarry in Gozo, Malta
-
Limestone as building material
-

Limestone is used worldwide as building textile.
Limestone is the raw material for production of lime, primarily known for treating soils, purifying water and smelting copper. Lime is an important ingredient used in chemical industries.[110] Limestone and (to a lesser extent) marble are reactive to acid solutions, making acrid rain a pregnant trouble to the preservation of artifacts made from this stone. Many limestone statues and building surfaces have suffered astringent impairment due to acrid pelting.[111] [112] Also limestone gravel has been used to protect lakes vulnerable to acid rain, acting every bit a pH buffering amanuensis.[113] Acid-based cleaning chemicals can too etch limestone, which should only be cleaned with a neutral or mild brine-based cleaner.[114]

A limestone plate with a negative map of Moosburg in Bavaria is prepared for a lithography print.

Plastic bag "made mainly from limestone"
Other uses include:
- It is the raw material for the manufacture of quicklime (calcium oxide), slaked lime (calcium hydroxide), cement and mortar.[58]
- Pulverized limestone is used equally a soil conditioner to neutralize acidic soils (agricultural lime).[115]
- Is crushed for apply equally amass—the solid base for many roads as well every bit in asphalt concrete.[58]
- As a reagent in flue-gas desulfurization, where it reacts with sulfur dioxide for air pollution control.[116]
- In glass making, peculiarly in the manufacture of soda-lime glass.[117]
- As an additive toothpaste, paper, plastics, paint, tiles, and other materials every bit both white paint and a inexpensive filler.[118]
- As rock dust, to suppress methane explosions in surreptitious coal mines.[119]
- Purified, it is added to breadstuff and cereals every bit a source of calcium.[120]
- As a calcium supplement in livestock feed, such as for poultry (when footing upwards).[121]
- For remineralizing and increasing the alkalinity of purified water to prevent pipage corrosion and to restore essential nutrient levels.[122]
- In blast furnaces, limestone binds with silica and other impurities to remove them from the atomic number 26.[123]
- It tin can help in the removal of toxic components created from coal called-for plants and layers of polluted molten metals.[124]
Many limestone formations are porous and permeable, which makes them of import petroleum reservoirs.[125] About 20% of North American hydrocarbon reserves are found in carbonate rock. Carbonate reservoirs are very mutual in the petroleum-rich Heart East,[58] and carbonate reservoirs hold about a third of all petroleum reserves worldwide.[126] Limestone formations are as well mutual sources of metallic ores, because their porosity and permeability, together with their chemical action, promotes ore deposition in the limestone. The lead-zinc deposits of Missouri and the Northwest Territories are examples of ore deposits hosted in limestone.[58]
Scarcity [edit]
Limestone is a major industrial raw material that is in constant demand. This raw fabric has been essential in the fe and steel manufacture since the nineteenth century.[127] Companies have never had a shortage of limestone; however, information technology has become a business organization as the demand continues to increase[128] and information technology remains in high demand today.[129] The major potential threats to supply in the nineteenth century were regional availability and accessibility.[127] The two main accessibility issues were transportation and holding rights. Other issues were high uppercase costs on plants and facilities due to environmental regulations and the requirement of zoning and mining permits.[103] These two dominant factors led to the adaptation and selection of other materials that were created and formed to design alternatives for limestone that suited economic demands.[127]
Limestone was classified as a critical raw material, and with the potential take chances of shortages, it drove industries to find new alternative materials and technological systems. This allowed limestone to no longer be classified as critical as replacement substances increased in production; minette ore is a mutual substitute, for example.[127]
Occupational safety and health [edit]
| NFPA 704 burn down diamond | |
|---|---|
| [130] 1 0 0 Limestone |
Powdered limestone as a food additive is generally recognized as safe[131] and limestone is not regarded as a hazardous textile. Even so, limestone dust tin can exist a mild respiratory and peel irritant, and grit that gets into the eyes can cause corneal abrasions. Because limestone contains small amounts of silica, inhalation of limestone dust could potentially lead to silicosis or cancer.[130]
Usa [edit]
The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for limestone exposure in the workplace as fifteen mg/m3 full exposure and 5 mg/miii respiratory exposure over an eight-hour workday. The National Found for Occupational Safety and Health (NIOSH) has gear up a recommended exposure limit (REL) of 10 mg/grand3 total exposure and 5 mg/m3 respiratory exposure over an 8-60 minutes workday.[132]
Graffiti [edit]
Removing graffiti from weathered limestone is difficult because it is a porous and permeable fabric. The surface is frail so usual abrasion methods run the risk of severe surface loss. Because it is an acid-sensitive stone some cleaning agents cannot exist used due to adverse effects.[133]
Gallery [edit]
-
A stratigraphic department of Ordovician limestone exposed in central Tennessee, U.S. The less-resistant and thinner beds are equanimous of shale. The vertical lines are drill holes for explosives used during road construction.
-

-

Fossils in limestone from the northern Black Sea region
-
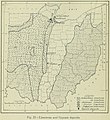
Limestone distribution in Ohio, from "Geography of Ohio," 1923
-

Chalk is a variety of limestone. Information technology is a softer, and more than powdery cloth.
See too [edit]
- Coral sand
- In Praise of Limestone – Poem by Westward. H. Auden
- Kurkar – Regional name for an aeolian quartz calcrete on the Levantine coast
- Limepit – Former method of calcining limestone
- Sandstone – Type of sedimentary rock
References [edit]
- ^ Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, Due north.J.: Pearson Prentice Hall. pp. 177, 181. ISBN0131547283.
- ^ Leong, Goh Cheng (27 October 1995). Certificate Physics And Man Geography; Indian Edition. Oxford University Press. p. 62. ISBN978-0-xix-562816-half-dozen.
- ^ a b c d due east f Boggs 2006, p. 159.
- ^ Blatt, Harvey; Tracy, Robert J. (1996). Petrology : igneous, sedimentary, and metamorphic (second ed.). New York: W.H. Freeman. pp. 295–300. ISBN0716724383.
- ^ Jackson, Julia A., ed. (1997). "Magnesian limestone". Glossary of geology (Quaternary ed.). Alexandria, Virginia: American Geological Constitute. ISBN0922152349.
- ^ Blatt, Harvey; Middleton, Gerard; Murray, Raymond (1980). Origin of sedimentary rocks (2d ed.). Englewood Cliffs, N.J.: Prentice-Hall. pp. 446, 510–531. ISBN0136427103.
- ^ Boggs 2006, p. 182-194.
- ^ Blatt, Middleton & Murray 1980, p. 448-449.
- ^ a b c d Blatt & Tracy 1996, p. 295.
- ^ Boggs 2006, p. 160.
- ^ Blatt, Middleton & Murray 1980, p. 467.
- ^ Blatt & Tracy 1996, pp. 301–302.
- ^ Oates, Tony (17 September 2010). "Lime and Limestone". Kirk-Othmer Encyclopedia of Chemical Engineering: 1209130507212019.a01.pub3. doi:x.1002/0471238961.1209130507212019.a01.pub3. ISBN978-0471238966.
- ^ "Compressive strength test". Encyclopedia Britannica . Retrieved 4 February 2021.
- ^ Blatt & Tracy 1996, pp. 295–296.
- ^ Blatt, Middleton & Murray 1980, p. 452.
- ^ a b Blatt & Tracy 1996, pp. 295–300.
- ^ Blatt, Middleton & Murray 1980, p. 449.
- ^ Boggs 2006, p. 161-164.
- ^ a b Blatt et al. sfn fault: no target: CITEREFBlattTracy1996297-299 (aid)
- ^ Boggs 2006, pp. 164–165.
- ^ Adachi, Natsuko; Ezaki, Yoichi; Liu, Jianbo (February 2004). "The fabrics and origins of peloids immediately after the end-Permian extinction, Guizhou Province, South China". Sedimentary Geology. 164 (1–2): 161–178. Bibcode:2004SedG..164..161A. doi:x.1016/j.sedgeo.2003.10.007.
- ^ a b c Blatt & Tracy 1996, p. 298.
- ^ Chafetz, Henry S. (1986). "Marine Peloids: A Production of Bacterially Induced Precipitation of Calcite". SEPM Journal of Sedimentary Research. 56 (vi): 812–817. doi:10.1306/212F8A58-2B24-11D7-8648000102C1865D.
- ^ Samankassou, Elias; Tresch, Jonas; Strasser, André (26 November 2005). "Origin of peloids in Early Cretaceous deposits, Dorset, South England" (PDF). Facies. 51 (one–4): 264–274. doi:10.1007/s10347-005-0002-8. S2CID 128851366.
- ^ Blatt & Tracy 1996, p. 299-300, 304.
- ^ Blatt, Middleton & Murray 1980, p. 460.
- ^ a b c Blatt & Tracy 1996, p. 300.
- ^ Boggs 2006, p. 166.
- ^ a b c Trower, Elizabeth J.; Lamb, Michael P.; Fischer, Woodward W. (16 March 2019). "The Origin of Carbonate Mud". Geophysical Research Messages. 46 (v): 2696–2703. Bibcode:2019GeoRL..46.2696T. doi:10.1029/2018GL081620. S2CID 134970335.
- ^ a b Boggs 2006, pp. 166–167.
- ^ Blatt & Tracy 1996, pp. 315–317.
- ^ Fratini, Fabio; Pecchioni, Elena; Cantisani, Emma; Antonelli, Fabrizio; Giamello, Marco; Lezzerini, Marco; Canova, Roberta (December 2015). "Portoro, the black and aureate Italian "marble"". Rendiconti Lincei. 26 (4): 415–423. doi:10.1007/s12210-015-0420-7. S2CID 129625906.
- ^ Blatt & Tracy 1996, pp. 474.
- ^ "Carbonate Classification: SEPM STRATA".
- ^ Folk, R. L. (1974). Petrology of Sedimentary Rocks. Austin, Texas: Hemphill Publishing. ISBN0-914696-14-nine.
- ^ Dunham, R. J. (1962). "Nomenclature of carbonate rocks according to depositional textures". In Ham, Westward. E. (ed.). Nomenclature of Carbonate Rocks. American Association of Petroleum Geologists Memoirs. Vol. 1. pp. 108–121.
- ^ Wright, Five.P. (1992). "A revised Classification of Limestones". Sedimentary Geology. 76 (3–4): 177–185. Bibcode:1992SedG...76..177W. doi:10.1016/0037-0738(92)90082-3.
- ^ Blatt, Middleton & Murray 1980, p. 479-480.
- ^ a b Boggs 2006, p. 172.
- ^ Boggs 2006, p. 177.
- ^ Boggs 2006, pp. 174–176.
- ^ Morse, John W.; Mackenzie, F.T. (1990). Geochemistry of sedimentary carbonates. Amsterdam: Elsevier. p. 217. ISBN9780080869629.
- ^ Boggs 2006, pp. 176–182.
- ^ Jerry Lucia, F. (September 2017). "Observations on the origin of micrite crystals". Marine and Petroleum Geology. 86: 823–833. doi:10.1016/j.marpetgeo.2017.06.039.
- ^ Blatt, Middleton & Murray 1980, pp. 460–464.
- ^ Boggs 2006, p. 180.
- ^ Boggs 2006, pp. 177, 181.
- ^ Blatt, Middleton & Murray 1980, pp. 497–501.
- ^ Blatt, Middleton & Murray 1980, p. 497-503.
- ^ Blatt & Tracy 1996, p. 312.
- ^ Blatt, Middleton & Murray 1980, pp. 507–509.
- ^ a b Blatt & Tracy 1996, p. 312-316.
- ^ a b Boggs 2006, pp. 186–187.
- ^ a b Machel, Hans G. (2004). "Concepts and models of dolomitization: a critical reappraisal". Geological Social club, London, Special Publications. 235 (1): seven–63. Bibcode:2004GSLSP.235....7M. doi:x.1144/GSL.SP.2004.235.01.02. S2CID 131159219.
- ^ a b Blatt, Middleton & Murray 1980, pp. 512–528.
- ^ Luczaj, John A. (Nov 2006). "Testify against the Dorag (mixing-zone) model for dolomitization along the Wisconsin arch ― A case for hydrothermal diagenesis". AAPG Bulletin. 90 (eleven): 1719–1738. doi:10.1306/01130605077.
- ^ a b c d e Blatt, Middleton & Murray 1980, p. 445.
- ^ Blatt, Middleton & Murray 1980, p. 448.
- ^ Boggs 2006, p. 159-161.
- ^ Boggs 2006, p. 176-177.
- ^ Blatt, Middleton & Murray 1980, p. 446, 733.
- ^ Blatt, Middleton & Murray 1980, p. 468-470.
- ^ Blatt, Middleton & Murray 1980, p. 446-447.
- ^ Blatt & Tracy 1996, p. 306-307.
- ^ Blatt, Middleton & Murray 1980, p. 474-479.
- ^ Blatt & Tracy 1996, p. 308-309.
- ^ Roeser, Patricia; Franz, Sven O.; Litt, Thomas (ane Dec 2016). "Aragonite and calcite preservation in sediments from Lake Iznik related to lesser lake oxygenation and water column depth". Sedimentology. 63 (seven): 2253–2277. doi:x.1111/sed.12306. ISSN 1365-3091. S2CID 133211098.
- ^ Blatt, Middleton & Murray 1980, p. 480-482.
- ^ a b Blatt & Tracy 1996, p. 309-310.
- ^ Trewin, Due north. H.; Davidson, R. G. (1999). "Lake-level changes, sedimentation and faunas in a Middle Devonian bowl-margin fish bed". Journal of the Geological Society. 156 (3): 535–548. Bibcode:1999JGSoc.156..535T. doi:10.1144/gsjgs.156.3.0535. S2CID 131241083.
- ^ "Term 'evaporite'". Oilfield Glossary. Archived from the original on 31 January 2012. Retrieved 25 November 2011.
- ^ Boggs 2006, p. 662.
- ^ Blatt, Middleton & Murray 1980, pp. 446, 471–474.
- ^ Blatt, Middleton & Murray 1980, pp. 446–471.
- ^ Blatt & Tracy 1996, p. 304.
- ^ Ehrlich, Henry Lutz; Newman, Dianne G. (2009). Geomicrobiology (5th ed.). pp. 181–182. ISBN9780849379079. Archived from the original on 10 May 2016.
- ^ Blatt & Tracy 1996, p. 307.
- ^ Pratt, Brian R. (1995). "The origin, biota, and development of deep-water mud-mounds". Spec. Publs Int. Ass. Sediment. 23: 49–123. ISBN9781444304121 . Retrieved 4 February 2021.
- ^ a b Blatt & Tracy 1996, pp. 307–308.
- ^ Riding, Robert (July 2002). "Structure and composition of organic reefs and carbonate mud mounds: concepts and categories". World-Science Reviews. 58 (1–2): 163–231. Bibcode:2002ESRv...58..163R. doi:10.1016/S0012-8252(01)00089-vii.
- ^ Wood, Rachel (1999). Reef evolution. Oxford: Oxford Academy Press. ISBN0198577842 . Retrieved 5 February 2021.
- ^ McGhee, George R. (2013). When the invasion of country failed : the legacy of the Devonian extinctions. New York: Columbia University Press. p. 101. ISBN9780231160575.
- ^ a b Thornbury, William D. (1969). Principles of geomorphology (2d ed.). New York: Wiley. pp. 303–344. ISBN0471861979.
- ^ "Karst Landscapes of Illinois: Dissolving Boulder and Collapsing Soil". Prairie Research Plant. Illinois State Geological Survey. Retrieved 26 December 2020.
- ^ Taylor, P. D.; Wilson, M. A. (2003). "Palaeoecology and evolution of marine difficult substrate communities" (PDF). Earth-Science Reviews. 62 (i–ii): 1–103. Bibcode:2003ESRv...62....1T. doi:10.1016/S0012-8252(02)00131-9. Archived from the original (PDF) on 25 March 2009.
- ^ Rodrı́guez-Vidal, J.; Cáceres, L.M.; Finlayson, J.C.; Gracia, F.J.; Martı́nez-Aguirre, A. (October 2004). "Neotectonics and shoreline history of the Rock of Gibraltar, southern Iberia". 4th Science Reviews. Elsevier (2004). 23 (18–19): 2017–2029. Bibcode:2004QSRv...23.2017R. doi:10.1016/j.quascirev.2004.02.008. Retrieved 23 June 2016.
- ^ McNamara, Grand.; Hennessy, R. (2010). "The geology of the Burren region, Co. Clare, Ireland" (PDF). Projection NEEDN, The Burren Connect Project. Ennistymon: Clare Canton Quango. Retrieved iii February 2021.
- ^ "Island of Wight, Minerals" (PDF). Archived from the original (PDF) on two November 2006. Retrieved eight October 2006.
- ^ Juerges, A.; Hollis, C. Due east.; Marshall, J.; Crowley, S. (May 2016). "The control of basin evolution on patterns of sedimentation and diagenesis: an example from the Mississippian Nifty Orme, North Wales". Journal of the Geological Society. 173 (3): 438–456. Bibcode:2016JGSoc.173..438J. doi:10.1144/jgs2014-149.
- ^ Cruslock, Eva M.; Naylor, Larissa A.; Foote, Yolanda Fifty.; Swantesson, Jan O.H. (Jan 2010). "Geomorphologic equifinality: A comparison betwixt shore platforms in Höga Kusten and Fårö, Sweden and the Vale of Glamorgan, South Wales, Uk". Geomorphology. 114 (one–two): 78–88. Bibcode:2010Geomo.114...78C. doi:ten.1016/j.geomorph.2009.02.019.
- ^ Luczaj, John A. (2013). "Geology of the Niagara Escarpment in Wisconsin". Geoscience Wisconsin. 22 (1): one–34. Retrieved 5 February 2021.
- ^ Miller, James F. (1969). "Conodont Fauna of the Notch Peak Limestone (Cambro-Ordovician), Business firm Range, Utah". Journal of Paleontology. 43 (2): 413–439. JSTOR 1302317.
- ^ Tran Duc Thanh; Waltham Tony (1 September 2001). "The outstanding value of the geology of Ha Long Bay". Advances in Natural Sciences. ii (3). ISSN 0866-708X.
- ^ Waltham, Tony (2010). Migon, Piotr (ed.). Guangxi Karst: The Fenglin and Fengcong Karst of Guilin and Yangshuo, in Geomorphological Landscapes of the Globe. Springer. pp. 293–302. ISBN9789048130542.
- ^ Mitchell-Tapping, Hugh J. (Spring 1980). "Depositional History of the Oolite of the Miami Limestone Formation". Florida Scientist. 43 (2): 116–125. JSTOR 24319647.
- ^ Thorsten Jansson, Stora Alvaret, Lenanders Tryckeri, Kalmar, 1999
- ^ Laufeld, S. (1974). Silurian Chitinozoa from Gotland. Fossils and Strata. Universitetsforlaget.
- ^ Pereira, Dolores; Tourneur, Francis; Bernáldez, Lorenzo; Blázquez, Ana García (2014). "Petit Granit: A Belgian limestone used in heritage, structure and sculpture" (PDF). Episodes. 38 (2): xxx. Bibcode:2014EGUGA..xvi...30P. Retrieved 5 Feb 2021.
- ^ a b Cassar, Joann (2010). "The utilise of limestone in historic context". In Smith, Bernard J. (ed.). Limestone in the Built Environment: Present-day Challenges for the Preservation of the Past. Geographical Society of London. pp. xiii–23. ISBN9781862392946. Archived from the original on xv Feb 2017.
- ^ Oates, J. A. (n.d.). Lime and limestone. Retrieved February 23, 2021, from https://books.google.ca/books?id=MVoEMNI5Vb0C&printsec=frontcover&dq=limestone%2Buses&hl=en&sa=X&ved=2ahUKEwje2dHc2YDvAhWviK0KHSb7CGcQ6AEwAHoECAQQAg#five=onepage&q=limestone%20uses&f=false
- ^ "Welcome to the Limestone Metropolis". Archived from the original on 20 February 2008. Retrieved 13 Feb 2008.
- ^ a b Corathers, L.A. (fifteen February 2019). "Lime". Metals and minerals: US Geological Survey Minerals Yearbook 2014, Volume 1. Washington, DC: USGS (published 2018). p. 43.ane. ISBN978-1-4113-4253-viii.
- ^ Cassar, Joann (2010). "The utilise of limestone in a celebrated context – the experience of Malta". Geological Society, London, Special Publications. 331 (1): 13–25. Bibcode:2010GSLSP.331...13C. doi:10.1144/SP331.two. S2CID 129082854.
- ^ Oates, J. A. (n.d.). Lime and limestone. Retrieved February 23, 2021, from https://books.google.ca/books?id=MVoEMNI5Vb0C&printsec=frontcover&dq=limestone%2Buses&hl=en&sa=10&ved=2ahUKEwje2dHc2YDvAhWviK0KHSb7CGcQ6AEwAHoECAQQAg#v=onepage&q=limestone%20uses&f=faux
- ^ Schele, Linda; Miller, Mary Ellen. The Claret of Kings: Dynasty and Ritual in Maya Fine art. Kimbell Fine art Museum. p. 41.
- ^ P. V. Sharma (1997), Ecology and Applied science Geophysics, Cambridge University Printing, p. 17, doi:10.1017/CBO9781139171168, ISBN9781139171168
- ^ "Odessa catacombs". Odessa travel guide. Retrieved 13 June 2020.
- ^ Ashurst, John; Dimes, Francis Yard. (1998). Conservation of building and decorative rock. Butterworth-Heinemann. p. 117. ISBN978-0-7506-3898-ii.
- ^ Bliss, J. D., Hayes, T. S., & Orris, G. J. (2012, Baronial). Limestone—A Crucial and Versatile Industrial Mineral Commodity. Retrieved February 23, 2021, from https://pubs.usgs.gov/fs/2008/3089/fs2008-3089.pdf
- ^ Reisener, A.; Stäckle, B.; Snethlage, R. (1995). "ICP on effects on materials". Water, Air, & Soil Pollution. 85 (four): 2701–2706. Bibcode:1995WASP...85.2701R. doi:10.1007/BF01186242. S2CID 94721996.
- ^ "Approaches in modeling the impact of air pollution-induced material degradation" (PDF). Archived from the original (PDF) on 16 July 2011. Retrieved 18 November 2010.
- ^ Clayton, Janet 50.; Dannaway, Eric Due south.; Menendez, Raymond; Rauch, Henry W.; Renton, John J.; Sherlock, Sean G.; Zurbuch, Peter E. (1998). "Application of Limestone to Restore Fish Communities in Acidified Streams". Northward American Journal of Fisheries Management. 18 (2): 347–360. doi:10.1577/1548-8675(1998)018<0347:AOLTRF>2.0.CO;2.
- ^ Hatch, Jonathan (eighteen Apr 2018). "How to clean limestone". How to Clean Things. Saint Paul Media, Inc. Retrieved v February 2021.
- ^ Oates, J. A. H. (11 July 2008). Lime and Limestone: Chemistry and Technology, Production and Uses. John Wiley & Sons. pp. 111–3. ISBN978-3-527-61201-7.
- ^ Gutiérrez Ortiz, F. J.; Vidal, F.; Ollero, P.; Salvador, L.; Cortés, V.; Giménez, A. (February 2006). "Airplane pilot-Plant Technical Assessment of Wet Flue Gas Desulfurization Using Limestone". Industrial & Engineering science Chemistry Inquiry. 45 (4): 1466–1477. doi:10.1021/ie051316o.
- ^ Kogel, Jessica Elzea (2006). Industrial Minerals & Rocks: Commodities, Markets, and Uses. SME. ISBN9780873352338. Archived from the original on sixteen December 2017.
- ^ Huwald, Eberhard (2001). "Calcium carbonate - pigment and filler". Calcium Carbonate: 160–170. doi:10.1007/978-three-0348-8245-3_7. ISBN978-3-0348-9490-6.
- ^ Human, C.K.; Teacoach, K.A. (2009). "How does limestone stone grit preclude coal grit explosions in coal mines?" (PDF). Mining Engineering: 61. Retrieved 30 November 2020.
- ^ "Why Fortified Flour?". Wessex Factory . Retrieved v February 2021.
- ^ "A Guide to Giving Your Layer Hens Plenty Calcium". Poultry 1. Archived from the original on iii Apr 2009.
- ^ "Nutrient minerals in drinking-water and the potential wellness consequences of consumption of demineralized and remineralized and contradistinct mineral content drinking-water: Consensus of the meeting". World Health Organization report. Archived from the original on 24 December 2007.
- ^ Tylecote, R. F. (1992). A history of metallurgy (2nd ed.). London: Institute of Materials. ISBN978-0901462886.
- ^ Elation, J. D., Hayes, T. S., & Orris, G. J. (2012, August). Limestone—A Crucial and Versatile Industrial Mineral Commodity. Retrieved February 23, 2021, from https://pubs.usgs.gov/fs/2008/3089/fs2008-3089.pdf
- ^ Archie, One thousand.Due east. (1952). "Classification of Carbonate Reservoir Rocks and Petrophysical Considerations". AAPG Bulletin. 36. doi:x.1306/3D9343F7-16B1-11D7-8645000102C1865D.
- ^ Boggs 2006, p. p=159.
- ^ a b c d Haumann, Due south. (2020). "Critical and scarce: the remarkable career of limestone 1850–1914". European Review of History: Revue européenne d'histoire. 27 (3): 273–293. doi:ten.1080/13507486.2020.1737651. S2CID 221052279.
- ^ Sparenberg, O.; Heymann, M. (2020). "Introduction: resource challenges and constructions of scarcity in the nineteenth and twentieth centuries". European Review of History: Revue européenne d'histoire. 27 (3): 243–252. doi:ten.1080/13507486.2020.1737653. S2CID 221055042.
- ^ ResearchAndMarkets.com (nine June 2020). "Global Limestone Market Analysis and Forecasts 2020-2027 - Steady Growth Projected over the Next Few Years - ResearchAndMarkets.com". Limestone - Global Market Trajectory & Analytics. businesswire.com. Retrieved 24 March 2021.
- ^ a b Lhoist Northward America. "Material Prophylactic Data Sheet: Limestone" (PDF) . Retrieved v February 2021.
- ^ "CFR - Code of Federal Regulations Championship 21". The states Food & Drug Administration. Usa Section of Wellness & Homo Services. Retrieved five February 2021.
- ^ "Limestone". NIOSH Pocket Guide to Chemic Hazards. CDC. Archived from the original on twenty Nov 2015. Retrieved nineteen November 2015.
- ^ Weaver, Martin Eastward. (Oct 1995). "Removing Graffiti from Celebrated Masonry". National Park Service. Retrieved 5 February 2019.
Further reading [edit]
| | Wikimedia Eatables has media related to Limestone. |
- Boynton, Robert S. (1980). Chemical science and Technology of Lime and Limestone. Wiley. ISBN0471027715.
Source: https://en.wikipedia.org/wiki/Limestone
Posted by: kennedyliaboarpood.blogspot.com





0 Response to "What Type Of Rock Forms When Heat And Pressure Change The Chemical Makeup Of A Rock"
Post a Comment